Which Coronavirus Vaccine Is In Phase 3
Nat Med 27 205211 2021. In 43548 participants 170 confirmed cases of COVID-19 were evaluated with 162 observed in the placebo group versus 8 in the vaccine group.
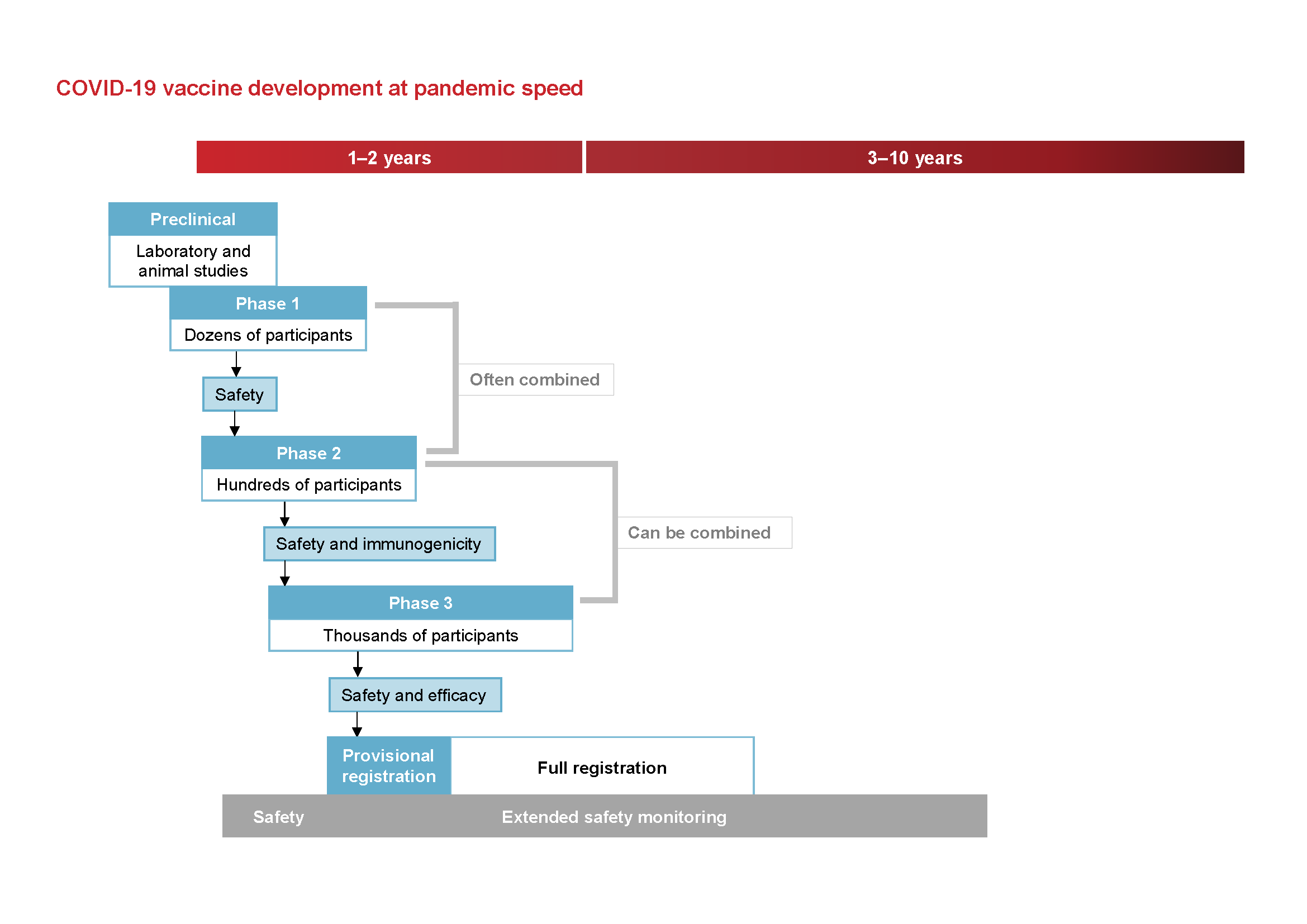
Phases Of Clinical Trials Ncirs
As the race to develop a safe and effective vaccine to protect against COVID-19 continues phase 3 trials of investigational vaccines are underway.
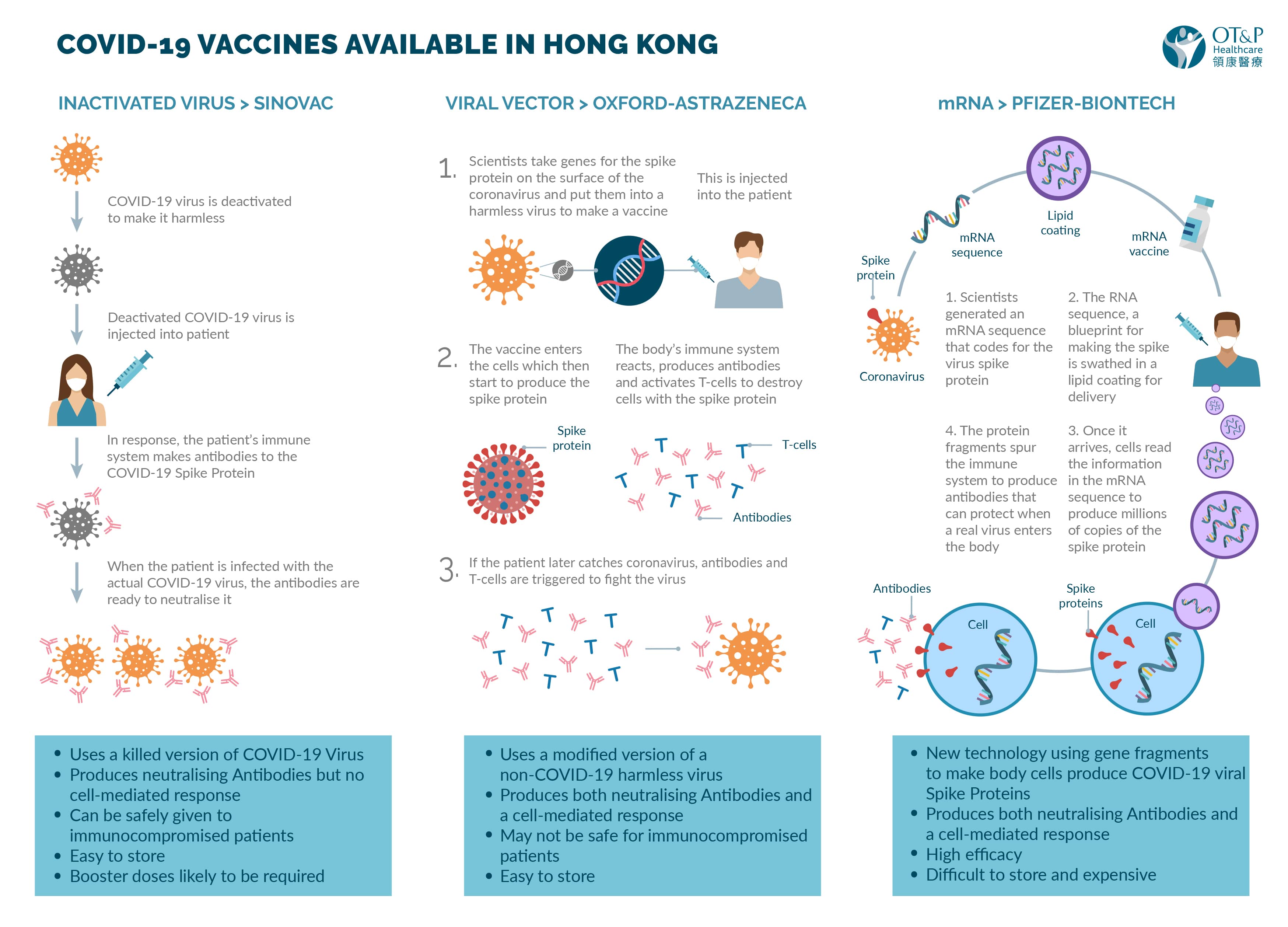
Which coronavirus vaccine is in phase 3. Our advice for clinicians on the coronavirus is here. AstraZeneca COVID-19 vaccine Novavax COVID-19 vaccine. Dine-in at restaurants are now allowed whereby the customers can only be allowed entry if they show their digital vaccination certificates.
With almost all individuals vaccinated Selangor confident of moving to Phase Two of NRP in Sept says MB Thursday 19 Aug 2021 0951 PM MYT Health workers administer Covid-19 vaccine doses at the Selangor Covid-19 Vaccination Programme at Dewan Seri Siantan in. Dine-ins allowed in Phase 1 states for fully vaccinated individuals. The delay comes due to the shortage of vaccine supply.
Gregory Poland an infectious diseases expert and head of Mayo Clinics Vaccine Research Group explains the meaning of phase 3 whos being tested and the hopeful outcomes. By Mohamed Basyir - August 19 2021 359am. If you are a member of the public looking for information and advice about coronavirus COVID-19 including information about the COVID-19 vaccine go to the NHS website.
Novavaxs two-dose COVID-19 vaccine showed 90 overall vaccine efficacy VE 100 protection against moderate and severe illness and 93 VE against variants of concern and of interest in a phase 3 US clinical trial in adults according to a company news release today. The government has announced more relaxations including dine. Kim JH Marks F.
Novavax on Monday announced the start of the Phase 3 trial of its Covid-19 vaccine in the United States and Mexico. On August 07 2021 the first volunteer enrolled in the Phase 3 clinical study of our COVID-19 vaccine candidate in Iran. The study is a randomized double-blind placebo-controlled trial and will evaluate the efficacy and safety of the vaccine in 16876 adult participants.
You can also find guidance and support on the GOVUK website. The publication of the primary. Seven COVID-19 vaccines in the third phase of clinical trials.
Pending positive Phase 3 outcomes and regulatory reviews the vaccine could be approved in Q4 2021 Today Sanofi and GlaxoSmithKline plc GSK started enrolment in their Phase 3 clinical study to assess the safety efficacy and immunogenicity of their adjuvanted recombinant-protein COVID-19 vaccine candidate. COVID-19 infection reduced by 95 in adult vaccine recipients according to Phase 3 trial results published in the New England Journal of Medicine. Looking beyond COVID-19 vaccine phase 3 trials.
TOKYO -- Daiichi Sankyo plans to begin Phase 3 clinical trials for its coronavirus vaccine this autumn with the aim of bringing it to the public in 2022 finally pushing forward Japans effort to. In my previous post I suggested that while were pursuing Phase 3 testing of several promising Covid-19 vaccines we could simultaneously offer those same unapproved vaccines to a wider. BNTX today announced that after conducting the final efficacy analysis in their ongoing Phase 3 study their mRNA-based COVID-19 vaccine candidate BNT162b2 met all of the studys primary efficacy endpoints.
Phase 3 of PICK was set to start in May 2021 covering a majority of the population until February 2022. It is the fifth company to launch a large-scale trial of a coronavirus vaccine. Delta variant now accounts for 1 in 4 cases nationwide Savannah Georgia CNN The first Phase 3 clinical trial of a coronavirus vaccine in.
While close to 200 vaccines are being developed for COVID-19 at least seven candidates have now moved to the phase 3 human trials - the final stage of testing to prove whether the vaccine is safe and effective against the virus before getting FDA approval. Vaccines in Phase 3 Clinical Trials As of February 27 2021 large-scale Phase 3 clinical trials are in progress or being planned for two COVID-19 vaccines in the United States. In this QA Dr.
NEW BRUNSWICK NJ April 21 2021 Johnson Johnson the Company today announced publication in the New England Journal of Medicine of primary data from the Phase 3 ENSEMBLE clinical trial for its single-dose COVID-19 vaccine developed by the Janssen Pharmaceutical Companies of Johnson Johnson Janssen. Basic principles of COVID-19 vaccination autumnwinter phase 3 which are designed to support your planning locally.

What Are The Phases Johnson County Kansas

Safety And Immunogenicity Of Chadox1 Ncov 19 Vaccine Administered In A Prime Boost Regimen In Young And Old Adults Cov002 A Single Blind Randomised Controlled Phase 2 3 Trial The Lancet
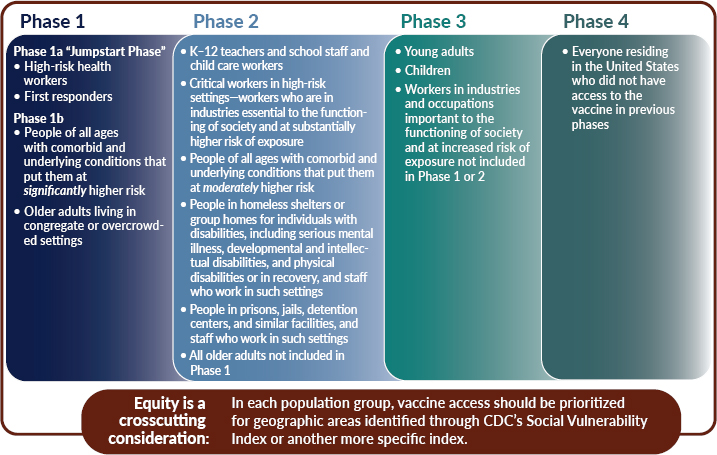
3 A Framework For Equitable Allocation Of Covid 19 Vaccine Framework For Equitable Allocation Of Covid 19 Vaccine The National Academies Press

Posting Komentar untuk "Which Coronavirus Vaccine Is In Phase 3"