Fda Approved Covid 19 Antibody Test Companies
The Food and Drug Administration FDA approves the use of five 5 Rapid Test Kits for COVID-19 today March 30 2020. The company submitted the application to the US.
Free Samples Wesail 15 Min Ce Ivd Covid 19 Antigen Test Kit
The Food and Drug Administration continues to expedite its approval time for technology that should help combat the coronavirus pandemicThis time the FDA cleared a coronavirus antibody test produced by Swiss diagnostics giant Roche for emergency use the company said Sunday.

Fda approved covid 19 antibody test companies. The tests are authorized in the United States for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection andor diagnosis of COVID-19 under Section 564b1 of the Act 21 USC. 519 rows The FDA issued a report on the use of additive manufacturing by non. This time the FDA cleared a coronavirus antibody test produced by Swiss diagnostics giant Roche for emergency use the company said Sunday.
Serological tests also called antibody tests detect antibodies to SARS-CoV-2. The test identifies via blood samples antibodies made. There are no currently available FDA approved COVID-19 test kits in the Philippines that differentiate the antibody protection gained from natural COVID-19 infection and the immunity from vaccination.
Has received Emergency Use Authorization EUA to use its proprietary AdexusDx product line for COVID-19 antibody testing in moderatecomplex laboratory settings and at the point of care in the US. 360bbb-3b1 unless the authorization is terminated or revoked sooner. A list of the local importers of the COVID-19 test kits with issued Special Certification is available upon request.
Springdale-based NOWDiagnostics Inc. Thus all companies who would require mass testing for their employees can purchase the COVID-19 test kits from these importers. Companies under fire for selling misbranded COVID-19 antibody test kits.
Dimension Vista SARS-CoV-2 Total antibody. The FDA supports all efforts to address this pandemic. The FDA reminds the public that at the present time there are no diagnostic or antibody COVID-19 test kits that are authorized cleared or approved to be used completely at home.
The test identifies via blood samples antibodies made by the body to fight off the coronavirus. Premier Biotech COVID-19 IgGIgM Rapid Test Cassette. CoronaCHEK COVID-19 IgGIgM Rapid Test Cassette.
FDA provides details about its thinking on antibody tests for COVID-19 and the agencys approach to making accurate and reliable serology tests widely available. Siemens Healthcare Diagnostics Inc. These point-of-care test kits are registered for use in countries with reliable regulatory agencies such as China and Singapore.
SARS-CoV-2 antibody often referred to as serology tests look for antibodies in a sample to determine if an individual has had a past infection with the virus that causes COVID-19. We approve kits that are registered and used in countries with advanced technology and. The FDA has already approved a number of COVID-19 test kits for local marketing.
COVID-19 Tests and Collection Kits Authorized by the FDA in 2020 FDAs Ongoing Work to Support and Advance COVID-19 Diagnostic Test Accuracy and. Current authorized antibody tests have not been evaluated to assess the level of protection provided by an immune response to COVID-19 vaccination. A Research Triangle Park-based company has received emergency-use authorization from the Food and Drug Administration for an antibody-based test it.
RxWiki News The US Food and Drug Administration FDA has sent warning letters to three companies for marketing COVID-19 antibody test kits with inaccurate or misleading claims. Food and Drug Administration FDA on May 29 2020. Orthos new quantitative COVID-19 IgG antibody test targets the S1 spike protein and is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2.
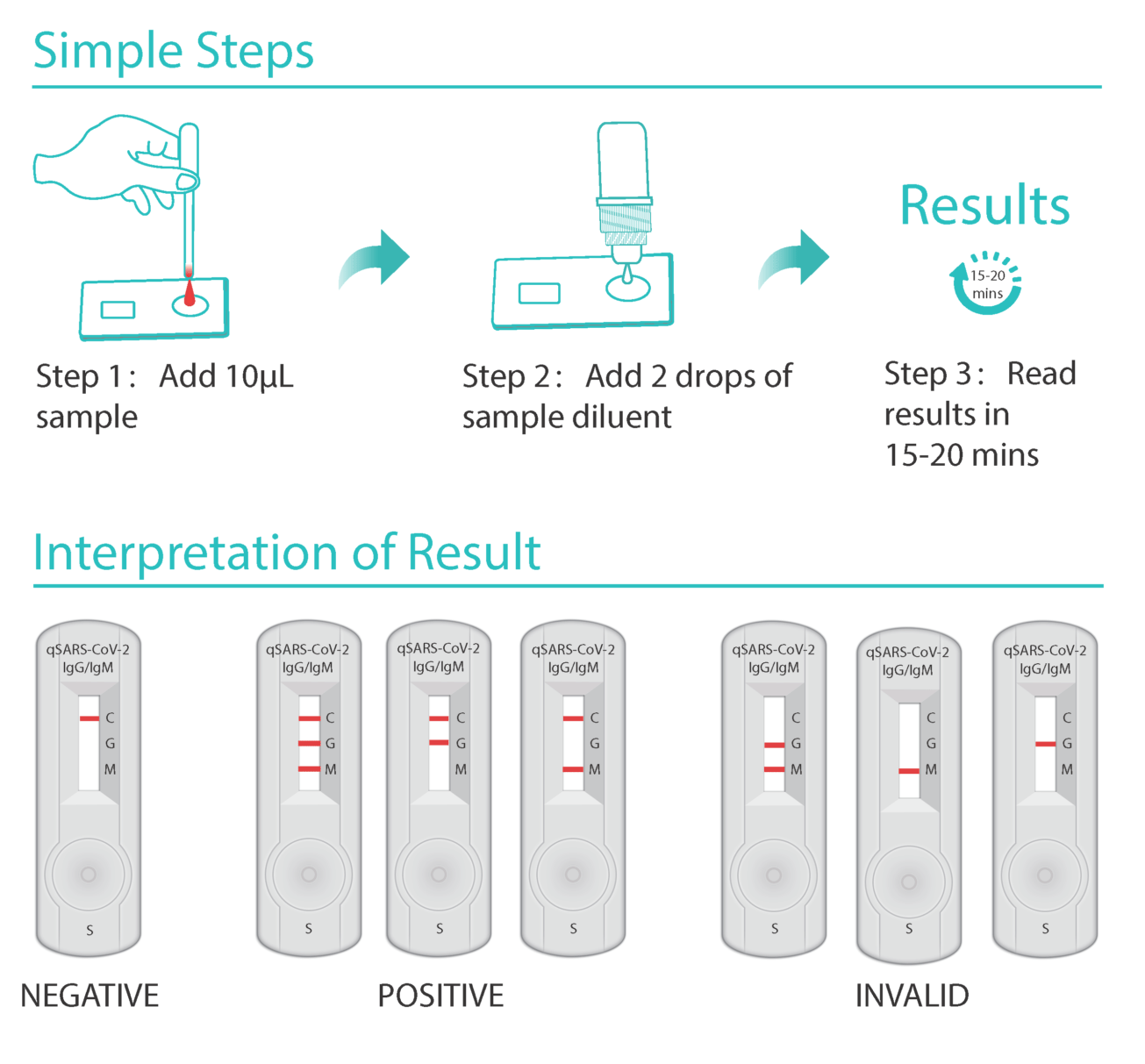
A Closer Look At Shortages Of Covid 19 Testing Kits In The Us And The Uk

New Covid 19 Test And A Decent First Quarter Buoy Abbott Evaluate

South Korean Ivd Company Sugentech S Covid 19 Igm Igg Rapid Test Listed On Fda
Siemens Healthineers Now Shipping Worldwide Total Antibody Test And Molecular Test Kit For Covid 19 With Expanded Capacity

Posting Komentar untuk "Fda Approved Covid 19 Antibody Test Companies"