Which Covid Vaccine Is In Phase 3
The publication of the primary analysis. They aim to test whether a vaccine is effective in preventing people from getting the disease in this case COVID-19.

Safety And Immunogenicity Of Chadox1 Ncov 19 Vaccine Administered In A Prime Boost Regimen In Young And Old Adults Cov002 A Single Blind Randomised Controlled Phase 2 3 Trial The Lancet
VLA a specialty vaccine company focused on the development and commercialization of prophylactic vaccines for infectious diseases with significant unmet medical need today announced the initiation of a further Phase 3 trial VLA2001-304 for its inactivated adjuvanted COVID-19 vaccine candidate.
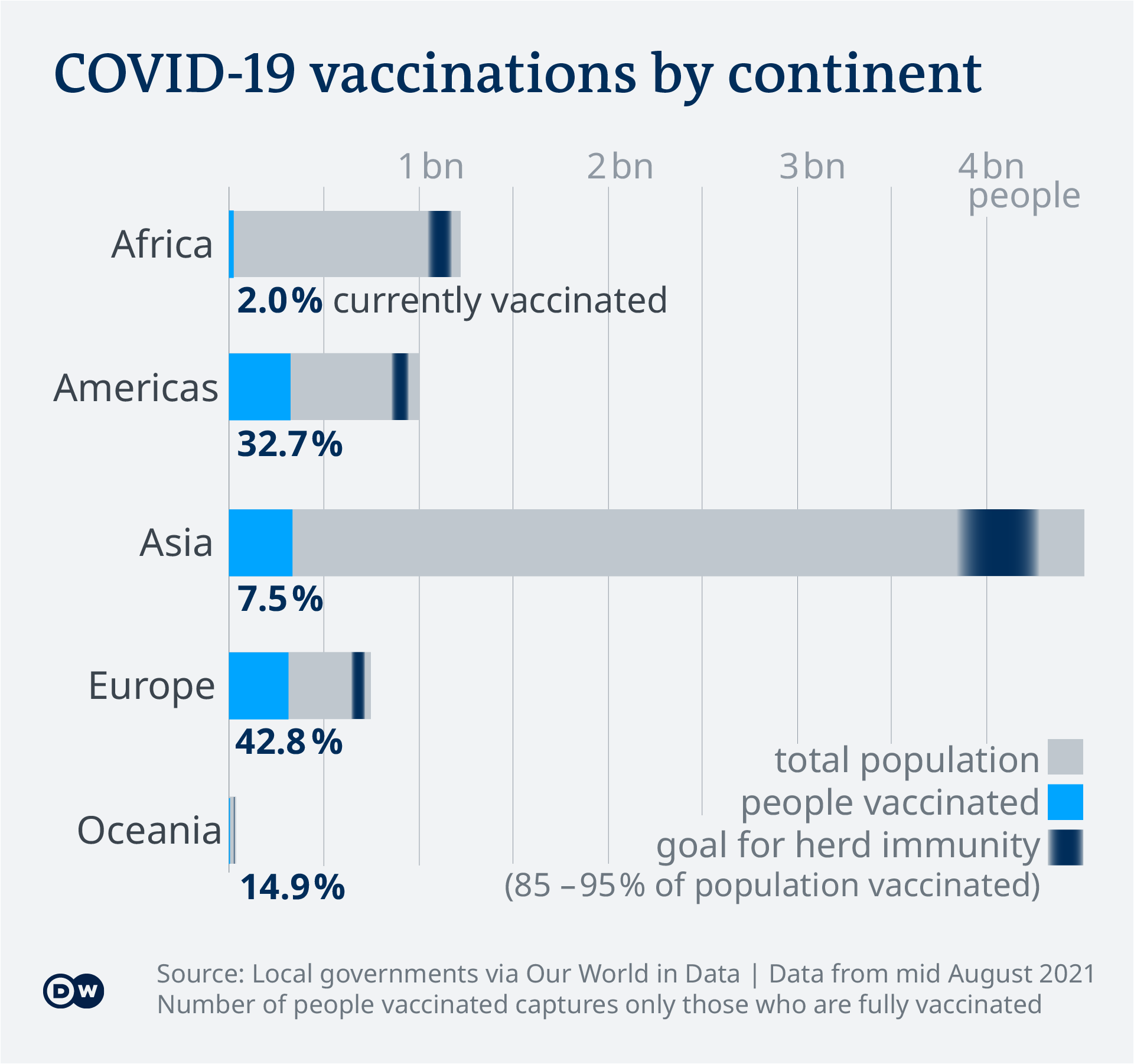
Which covid vaccine is in phase 3. Phase 3 clinical trial of COVID-19 vaccine underway August 6 2020 As the race to develop a safe and effective vaccine to protect against COVID-19 continues phase 3 trials of investigational vaccines are underway. Protein subunit vaccine Trial phase. Published 14 July 2021.
Abdala CIGB 66 is a protein vaccine that uses yeast as a receptor-binding domain RBD protein and alumina as an adjuvant. Letter from Dr Nikki Kanani and Caroline Temmink announcing publication of the Enhanced Service Specification for phase 3 of the COVID-19 vaccination programme and guidance on the General Practice Phase 3 Expression of Interest and Site Designation process. According to the ministry SK Bioscience will collect and analyze clinical data during the phase 3 study to see if the vaccine candidate is effective against the COVID-19 variants.
The first nasal vaccine against COVID-19 developed by Bharat Biotech has received regulators nod for conducting phase 2 and 3 clinical trials the Department of Biotechnology said on Friday. The phase three trials for ARCT-021 as the vaccine is called will be conducted in three yet-to-be disclosed locations where the populations have yet to be vaccinated. BNTX today announced that after conducting the final efficacy analysis in their ongoing Phase 3 study their mRNA-based COVID-19 vaccine candidate BNT162b2 met all of the studys primary efficacy endpoints.
NEW BRUNSWICK NJ April 21 2021 Johnson Johnson the Company today announced publication in the New England Journal of Medicine of primary data from the Phase 3 ENSEMBLE clinical trial for its single-dose COVID-19 vaccine developed by the Janssen Pharmaceutical Companies of Johnson Johnson Janssen. In a Phase 3 trial researchers usually compare data between vaccinated people and those who received a placebo like a salt water injection. The South Korean company reported promising interim data from its phase 12 trial.
Phase III Abdala is one of three vaccine candidates for Covid-19 being developed in-house by Cubas Center for Genetic Engineering and Biotechnology CIGB. Basic principles of COVID-19 vaccination autumnwinter phase 3 which are designed to support your planning locally. The third phase of Covid-19 vaccination drive targeted at senior citizens is likely to get underway anytime in March Health Minister Harsh Vardhan told the Lok Sabha on Friday.
Vaccines in Phase 3 Clinical Trials As of February 27 2021 large-scale Phase 3 clinical trials are in progress or being planned for two COVID-19 vaccines in the United States. Novovax has begun enrolling adult volunteers for a phase 3 trial of its COVID-19 vaccine candidate the fifth COVID-19 vaccine to reach phase 3 development in. Phase 1 clinical trial has been completed in age groups ranging from 18 to 60 years it said.
In the first stage of the. SeventyFour iStock The first full peer-reviewed results of phase 3 trials of the COVID-19 vaccine developed by AstraZeneca and Oxford University show that it is safe and up to 90 effective in preventing infection supporting regulatory submissions for emergency use. Гам-КОВИД-Вак is an adenovirus viral vector vaccine for COVID-19 developed by the Gamaleya Research Institute of Epidemiology and Microbiology in RussiaIt is the worlds first registered combination vector vaccine for the prevention of COVID-19 having been registered on 11 August 2020 by the Russian Ministry of Health.
Спутник V or Gam-COVID-Vac Russian. AstraZeneca COVID-19 vaccine Novavax COVID-19 vaccine. SAINT-HERBLAIN France I August 11 2021 I Valneva SE Nasdaq.
Phase 3 trials also thoroughly assess the vaccine for safety and side effects. SK bioscience has secured approval for phase 3 trials pitting its COVID-19 vaccine candidate GBP510 against AstraZenecas Vaxzevria. VLA2001-304 seeks to generate data in the elderly and is designed to potentially enable variant-bridging through immune-comparability.
The specialty vaccine firm Valneva SE has initiated a Phase 3 trial for its COVID-19 vaccine candidate VLA2001. For these reasons even when COVID-19 vaccines have achieved licensure via current phase 3 trials there will be substantial uncertainties about how useful the vaccines.
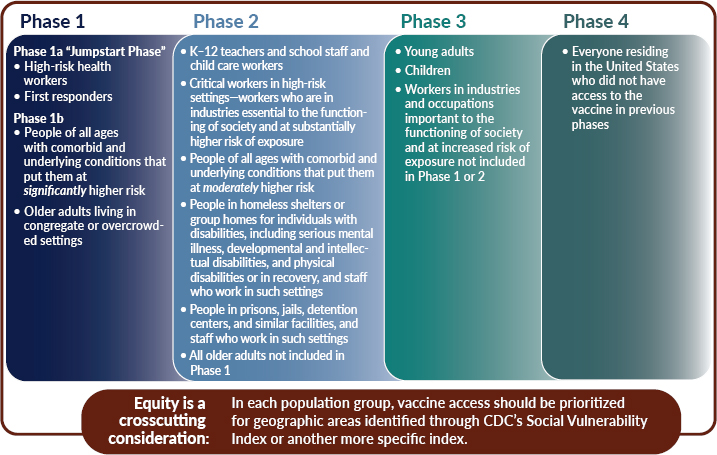
3 A Framework For Equitable Allocation Of Covid 19 Vaccine Framework For Equitable Allocation Of Covid 19 Vaccine The National Academies Press
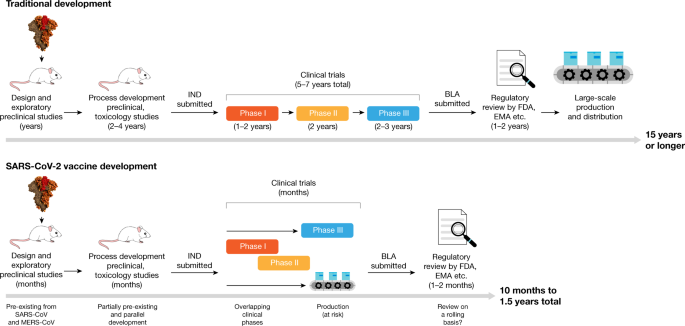
Sars Cov 2 Vaccines In Development Nature
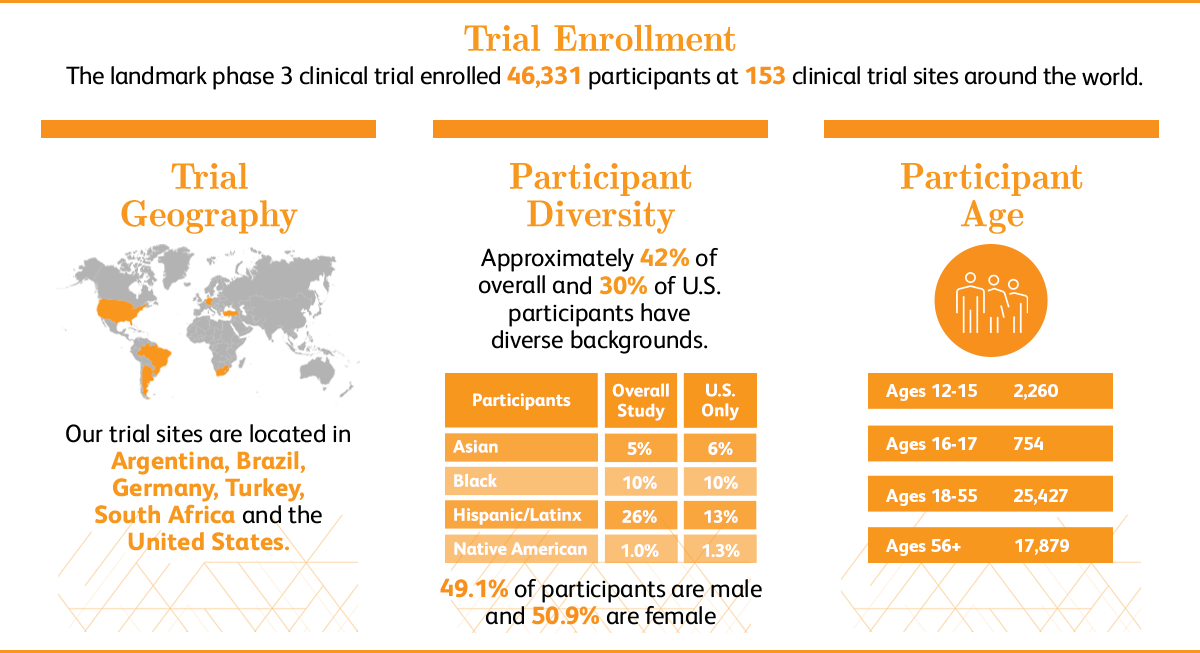
About Our Landmark Trial Pfpfizeruscom
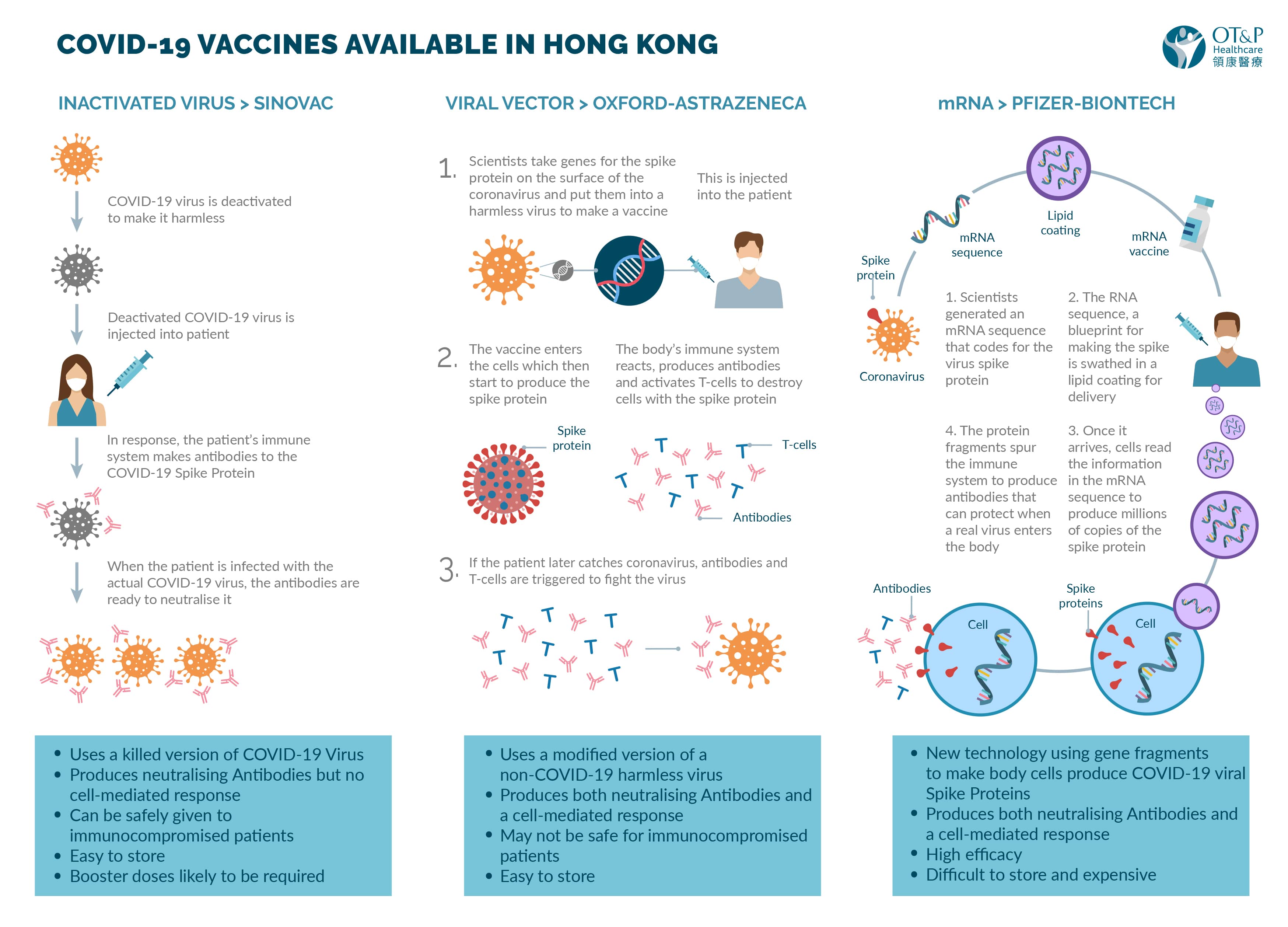
Posting Komentar untuk "Which Covid Vaccine Is In Phase 3"