Booster Dose Approval
Government has not approved booster shots against the virus saying it has yet to see evidence they are necessary. 11The US Food and Drug Administration is expected to announce within the next 48 hours that it is authorizing Covid-19 vaccine booster shots for people who are immunocompromised according to.
Pfizer Will Seek Approval For A Third Booster Dose Of Its Covid 19 Vaccine Independent Ie
16Pfizer is seeking the FDAs approval for a booster dose of its coronavirus vaccine as the pharma giant on Monday submitted data that shows robust immune responses from people who got.
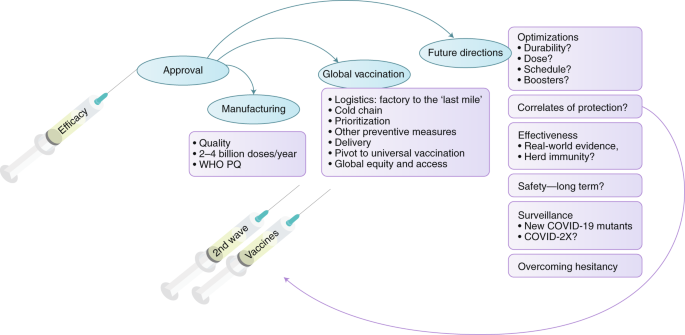
Booster dose approval. 11The Food and Drug Administration has not changed clinical vaccine guidelines to allow for extra doses though regulators are expected to recommend the so. It makes it the first coronavirus vaccine approved for individuals younger than 16 in Australia. 13The CDC adopted a recommendation from its Advisory Committee on Immunization Practices to clear Covid booster shots for people with weak.
1일 전The Empire State already approved a third dose of the Pfizer and Moderna vaccine for two-dose recipients who have weakened immune systems this week allowing them to get their booster. This is in addition to the already-approved third dose approved for some immunocompromised Americans. The pharma giant and its partner.
13In the US Mississippi a state with some of the lowest vaccination rates is advising that doctors consider a booster dose for people with weakened immune systems. On Thursday Pfizers Dr. Regulators to authorize a booster dose of its vaccine.
2일 전DAYTON Ohio WDTN A third booster shot for people who received their second dose eight months ago is currently pending FDA approval. According to Pfizer representatives the request is based on evidence from Israel and its own studies showing reduced efficacy six months after vaccination. 8But antibodies naturally wane over time so studies also are underway to tell if and when boosters might be needed.
7While Pfizer has said it plans to seek US. 25Pfizers COVID-19 vaccine has been approved for children aged 12 to 15 years. The discussion was sparked when Pfizer-BioNTech announced it would seek approval from US.
12Along with announcing plans to seek regulatory approval for a booster shot Pfizer and BioNTech cited data from Israels Ministry of Health showing vaccine efficacy in preventing both infection. Mikael Dolsten told The Associated Press that early data from the companys booster study suggests peoples antibody levels jump five- to 10-fold after a third dose compared to their second dose months earlier. People with severely weakened immune systems should be able to receive a third dose of.
MORESources say US to recommend COVID vaccine boosters at 8 months. Food and Drug Administration approval for booster shots health authorities say that for now the fully vaccinated seem well protected. 6Recommendations for booster doses of COVID-19 vaccines Policy on booster doses coordinated with FDA for possible amendments to EUA and ACIP for recommendations around use in specific populations Both will require data on safety immunogenicityand public health need.
13About 27M immunocompromised Americans CDC says. To Advise Boosters for Most Americans 8 Months After Vaccination Nursing home residents and health care workers will most likely be the first to get booster. 16Pfizer has submitted initial data to the FDA as it seeks approval for vaccine booster shots for all adults saying its trial showed third doses were more effective against Covid-19 and its more contagious Delta variant.
Likely to recommend booster shots eight months after second dose Pfizer and BioNTech said Monday they have submitted early stage clinical trial data to the Food and Drug Administration as part.
Pfizer Met With U S Agencies Approval Of A Covid 19 Booster Is No Closer Barron S
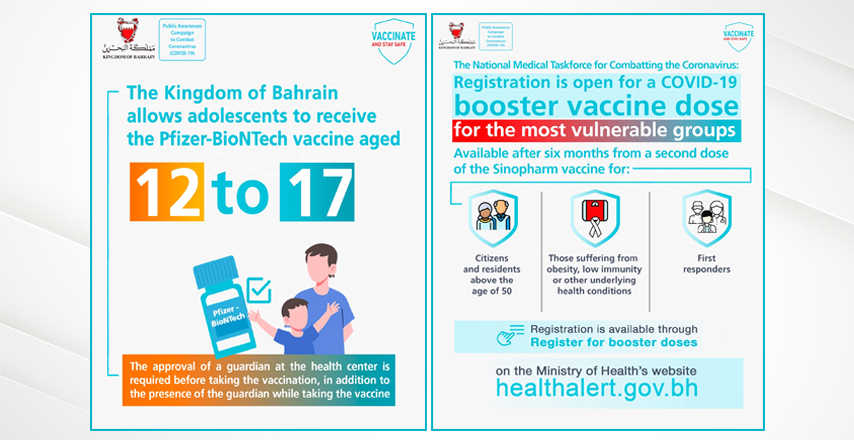
The Kingdom Of Bahrain Allows Vaccination For Adolescents Aged 12 To 17 And A Booster Dose For Those Over Fifty The Official Website For The Latest Health Developments Kingdom Of Bahrain

Some In U S Getting Covid 19 Booster Shot Without Fda Approval

The Kingdom Of Bahrain Allows Vaccination For Adolescents Aged 12 To 17 And A Booster Dose For Those Over Fifty

Posting Komentar untuk "Booster Dose Approval"