Fda Approved Covid 19 Antibody Tests
There are no currently available FDA approved COVID-19 test kits in the Philippines that differentiate the antibody protection gained from natural COVID-19 infection and the immunity from vaccination. In a recent post we warned readers against rushing out to purchase one of the many COVID-19 antibody tests suddenly flooding the market noting that few of these tests have been independently validated and many are grossly inaccurate.

Coronavirus Antibody Tests Fda Raises Standards Shots Health News Npr
2020-006 was approved allowing for the issuance of Special Certification for imported in-vitro diagnostic IVD kits used for diagnosis and screening of Coronavirus Disease 2019 COVID-19.

Fda approved covid 19 antibody tests. COVID-19 Tests and Collection Kits Authorized by the FDA in 2020. Antibody tests for COVID-19 received emergency use authorization EUA from the FDA in March. Health care providers should not expect serological antibody tests for coronavirus disease 2019 COVID-19 to definitively diagnose or exclude SARS-CoV-2 infection according to a letter from the Food and Drug Administration FDA.
The FDA is committed to helping ensure the public has access to a wide variety of test. In some validation studies of these tests like the one FDA is conducting in partnership with NIH CDC and BARDA the samples used in addition to. An implementation of this test the COVID-19 ELISA IgG Antibody Test has been granted an EUA authorization by FDA for use at the Mount Sinai Laboratory MSL Center for.
Text Version of Infographic. May 4 2020 Kim Schive May 4. Currently there are two types of diagnostic tests for COVID-19.
Am J Law Med. Home Quick Novel Coronavirus IgGIgM Antibody Test Kit with FDA Approved find complete details about Home Quick Novel Coronavirus IgGIgM Antibody Test Kit with FDA Approved Novel Coronavirus IgGIgM antibody test kit with FDA Approved Home Novel Coronavirus IgGIgM antibody test kit Quick Novel Coronavirus IgGIgM antibody test kit - Cofoe Medical Technology. Doctors say COVID-19 antibody tests arent FDA approved should be taken with caution Thousands of people are paying out of pocket to take an antibody test at private labs in the valley.
The FDAs Experience with Covid-19 Antibody Tests Early in the pandemic the FDA recognized that ensuring access to antibody tests could advance understanding of. Food and Drug Administration FDA recommends that clinical laboratories and health care providers stop using COVID-19 antibody tests that are listed on FDAs. In a letter received by the Food and Drug Administration FDA on 17 April 2018 RITM Director Dr.
This comes as relaxed rules by the agency have allowed untested and potentially unreliable tests for COVID-19 to enter the market something that may slow down the countrys recovery from the virus. The FDA already subjects most medical devices to much less stringent approval requirements than drugs and biologics and attempts to speed up rollout during the COVID crisis have been problematic. Austin-based medical technology company Babson Diagnostics has received emergency use authorization from the Food Drug Administration for its COVID-19 antibody test the company announced Thursday.
On 12 March 2020 Food and Drug Administration FDA Memorandum No. Celia Carlos informed FDA Director General Rolando Enrique Domingo that the Institute can now evaluate approved rapid antibody test kits in the interest of national emergency health and safety. Molecular RT-PCR tests which detect the virus genetic material.
Current authorized antibody tests have not been evaluated to assess the level of protection provided by an immune response to COVID-19 vaccination. Food and Drug Administration FDA is reminding the public and health care providers that results from currently authorized SARS-CoV-2 antibody tests. How accurate are FDA-approved COVID-19 antibody tests.
26 rows On June 16 2020 based on the FDAs continued review of the scientific. So far only 3 of at least 90 COVID-19 antibody tests in the US have been approved by the Food and Drug Administration FDA. 519 rows The FDA today announced it has issued an emergency use authorization EUA for the Symbiotica COVID-19 Self-Collected Antibody Test System the first antibody test.

Fda Advisory No 2020 483 Fda Approves Rapid Antibody Test Kits For Covid 19 Food And Drug Administration
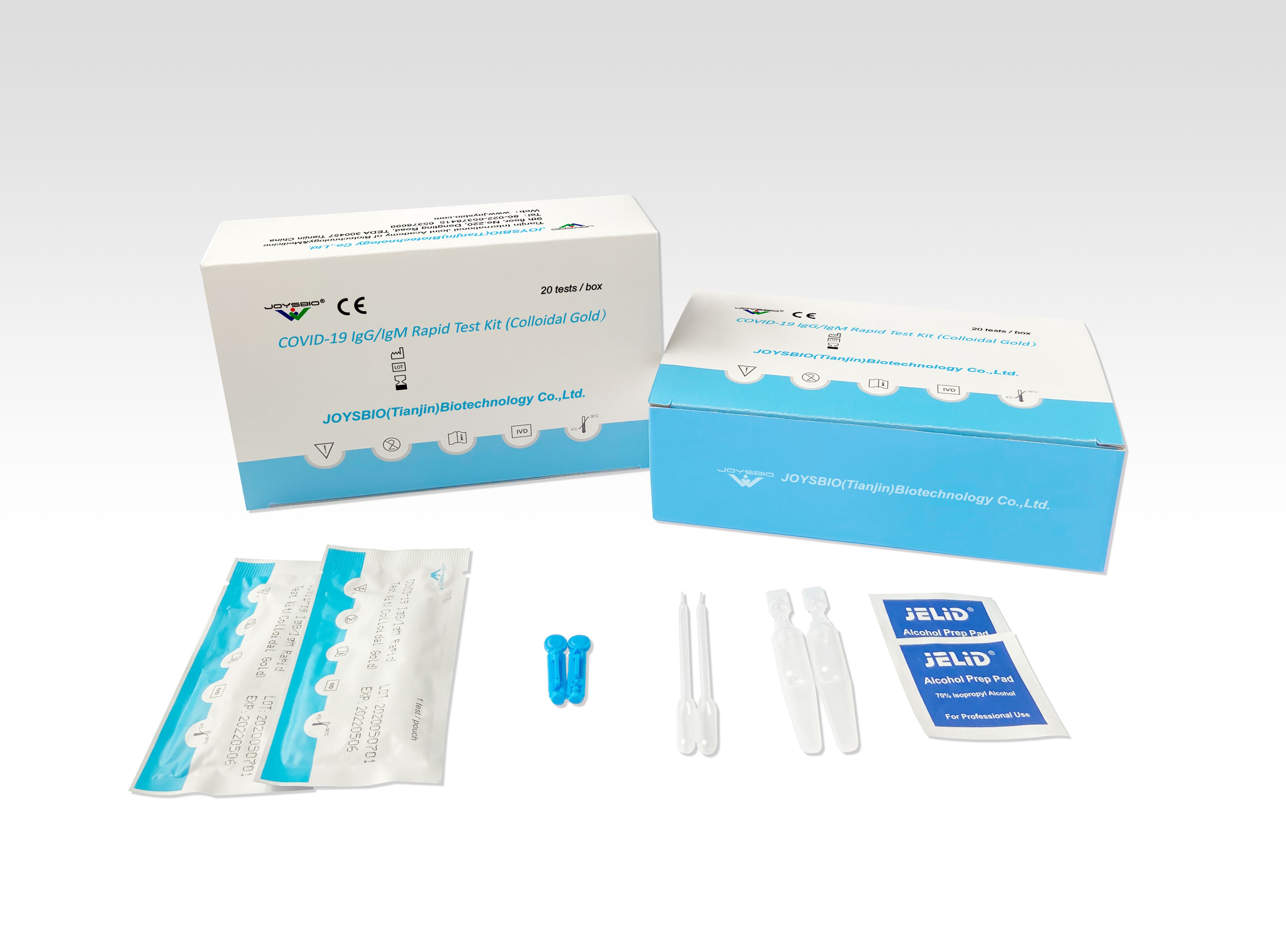
Covid 19 Antibody Rapid Test Kit Coronavirus Igg Igm Rapid Test

Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
Posting Komentar untuk "Fda Approved Covid 19 Antibody Tests"