Booster Dose Of Gardasil
Gardasil should be administered according to a 3-dose 05ml at 0 2 6months schedule. 6 months after first injection.
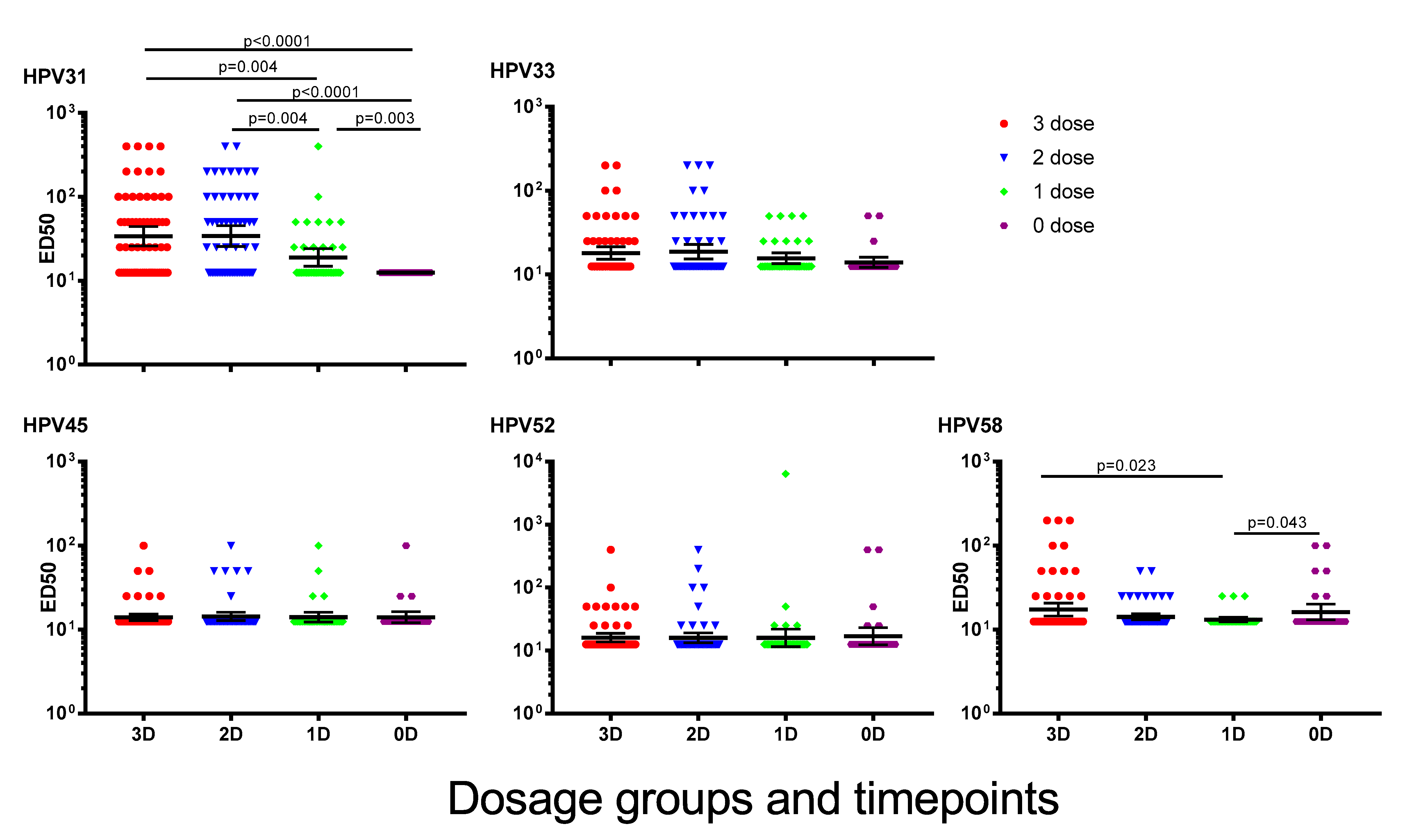
Vaccines Free Full Text Selective Persistence Of Hpv Cross Neutralising Antibodies Following Reduced Dose Hpv Vaccine Schedules Html
The second and third doses should be given at two and six months respectively after the first dose.
Booster dose of gardasil. 10Gardasil should be administered according to a 3-dose 05 ml at 0 2 6 months schedule. If the second shot is given less than 5 months after the first shot a third shot should be. 6 months after the first dose and not earlier than 3 months after the 2 nd dose.
366 out of 416 eligible girls participated in this follow. If youre a few days late getting your second or third dose of GARDASIL 9 dont. This randomized blinded study evaluated the.
Who Gets 2 Doses. 2 months after the first dose and not earlier than 1 month after the 1 st dose. Younger adolescents ages 9 and 10 and teens ages 13 and 14 also are able to receive vaccination on the updated two-dose schedule.
GARDASIL 9 should be administered intramuscularly in the deltoid or anterolateral area of the thigh. 14The Centers for Disease Control and Prevention CDC now recommends that all 11- and 12-year-olds receive two doses of HPV vaccine at least six months apart instead of the previously recommended three-dose schedule. You may have the first shot at any time as long as you are between the ages of 9 and 45 years.
The second dose should be administered at least one month after the first dose and the third dose should be administered at least 3 months after the second dose. 20If the second dose is administered earlier than 5 months after the first dose administer a third dose at least 4 months after the second dose. The second dose should be administered at least one month after the first dose and the third dose should be administered at least 3 months after the second dose.
The second dose should be administered at leastone month after the first dose and the third dose should be administered at least 3months after the second dose. 0 2 6 months. This randomized blinded study evaluated the immunogenicity and safety of a booster dose of Gardasil qHPV or Cervarix bHPV when administered to 12-13 year-old girls who were vaccinated at the age of 9-10 with 2 doses of qHPV 0-6 months.
0 2 6 months. But studies suggest that even if people dont. CDC recommends two doses of HPV vaccine for all adolescents at age 11 or 12 years.
A third dose may be given 6 to 12 months after your first shot. For persons 9 through 14 years of age GARDASIL 9 can be given using a 2-dose or 3-dose schedule. The second dose is given 2 to 6 months after your first shot.
Gardasil9 therefore extends the protection against disease caused by HPV. Gardasil9 includes the HPV types covered by 6 11 16 and 18 plus an additional five oncogenic HPV types 31 33 45 52 and 58. 2 months after first injection.
For the 2-dose schedule the second dose should be. For the 2-dose schedule the second shot should be given 612 months after the first shot. Our data suggest that this extensive swelling reaction may be more common with vaccines containing high diphtheria toxoid content.
The two doses of Gardasil9 should be administered 6 to 12 months apart. HPV vaccine may be given at the same time as other vaccines. 11The vaccine is given in a series of three injections over a six-month period.
0 6 to 12 months 3-dose. A 2-dose schedule is recommended for people who get the first dose before their 15 th birthday. Booster doses of DTaP vaccines can cause entire limb swelling which is usually associated with redness and pain.
30Gardasil9 replaces the 4-valent HPV vaccine Gardasil for which a 3-dose schedule has been used. See Clinical Studies 142 and 146 9 through 14 years. 15 through 45 years.
13Gardasil should be administered according to a 3-dose schedule. The need for a booster dose has not been established. 1This randomized blinded study evaluated the immunogenicity and safety of a booster dose of Gardasil qHPV or Cervarix bHPV when administered to 1213 year-old girls who were vaccinated at the age of 910 with 2 doses of qHPV 06 months.
For individuals 9 through 14 years of age GARDASIL 9 can be administered using a 2-dose or 3-dose schedule. 30Participants who received one booster dose of Gardasil 9 will be contacted to return to the clinic to provide blood specimens at 48 3 60 3 and 72 3 months after the priming dose. 7Gardasil 9 vaccine is given in a series of 2 or 3 shots.
At a date you and your healthcare provider choose. 30The CDC recommends people receive the second HPV dose one to two months after the first and receive the third dose six months after the first. Subjectspreviously vaccinated with a 3-dose regimen of quadrivalent HPV types6 11 16 and 18 vaccine Gardasil hereafter referred to as qHPVvaccine may receive 3doses of Gardasil9see.
In a 2-dose series the second dose should be given 612 months after the first dose 0 612 month schedule. Studies using a mixed regimen interchangeability of HPV vaccines were not performed for Gardasil9. All three doses should be.
Serologic geometric mean titers GMT of the nine vaccine types HPV 1618 6113133455258 will be measured at each time point.
Https Www Who Int Immunization Hpv Grad Booster Pdf

Hpv16 And Hpv18 Antibody Concentration Following Fewer Than Three Doses Download Scientific Diagram
Dosing Schedules And Administration Of Gardasil 9 Human Papillomavirus 9 Valent Vaccine Recombinant

Posting Komentar untuk "Booster Dose Of Gardasil"