What Coronavirus Antibody Tests Are Fda Approved
Widespread antibody testing can be. The Food and Drug Administration FDA reminds the public on the use of the different COVID-19 Antibody Test kits authorized for use in the Philippines.

Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
The COVID-19 infection initiates a humoral immune response that produces antibodies against specific viral antigens such as the Nucleocapsid protein N protein and Spike protein S protein.
What coronavirus antibody tests are fda approved. This new generation of test the. I could feel it feel my. The Food and Drug Administration is stiffening its rules to counteract what some have called a Wild West of antibody testing for the coronavirus.
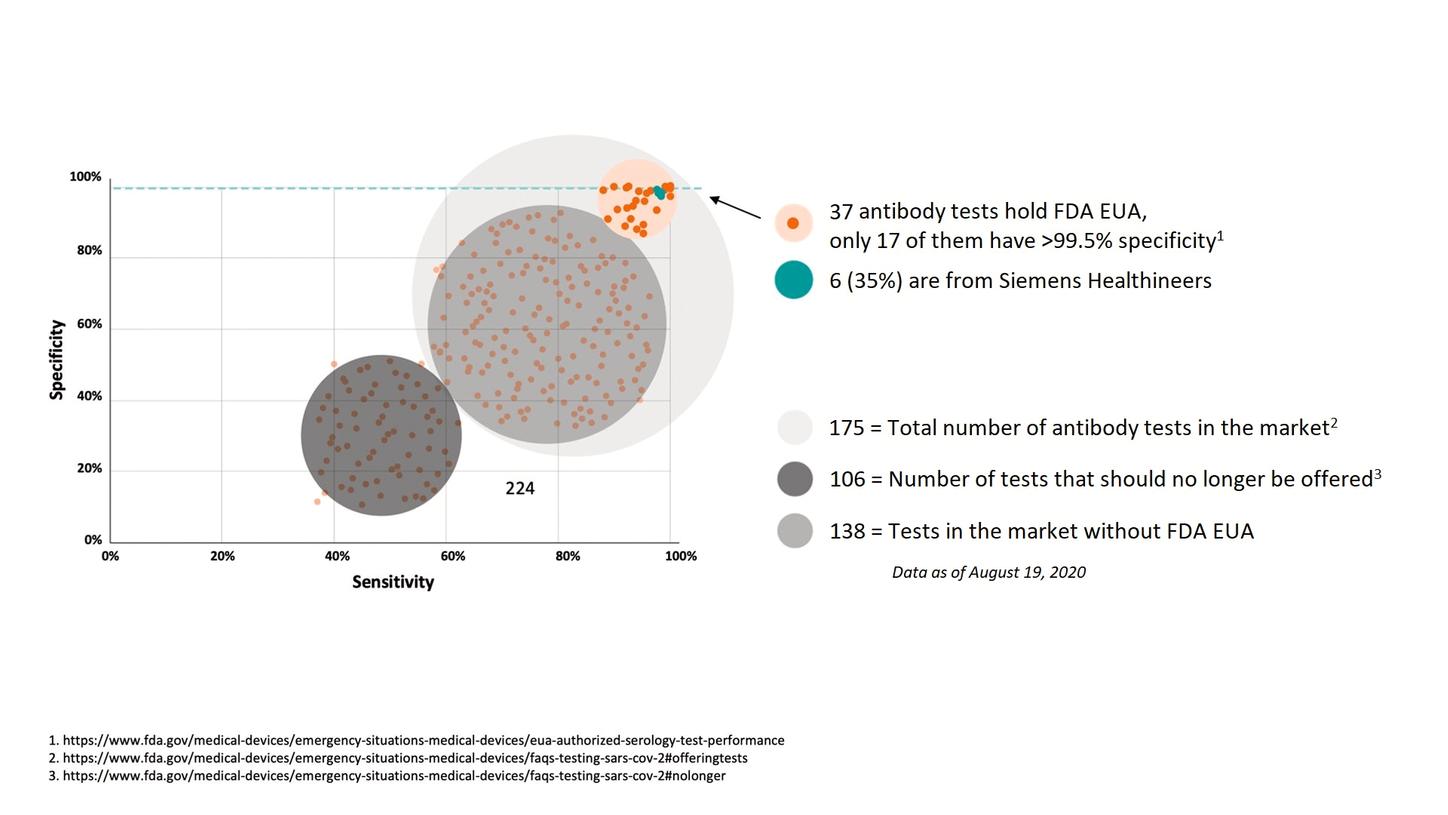
Infographic provides a visualization of data associated with CDRHs authorization of coronavirus COVID-19 Diagnostic Tests in 2020. On Wednesday July 28 the FDA revised the EUA for baricitinib sold under the brand name Olumiant now authorizing baricitinib alone for the treatment of COVID-19 in. The Food and Drug Administration FDA approves the use of five 5 Rapid Test Kits for COVID-19 today March 30 2020.
In Leigh Anns case it didnt. The US Food and Drug administration granted emergency approval for another two coronavirus blood tests both developed to determine who has already recovered from the fast-spreading illness. These point-of-care test kits are registered for use in countries with reliable.
Food and Drug Administration FDA is reminding the public and health care providers that results from currently authorized SARS-CoV-2 antibody tests. The FDAs Experience with Covid-19 Antibody Tests Jeffrey Shuren MD JD and Timothy Stenzel MD PhD. Designed to detect coronavirus antibodies according to a letter of authorization.
The lab results for antibody tests state it is unknown how long antibodies last and if the presence of antibodies means immunity. The Food and Drug Administration FDA has approved the first test in the US. The FDA supports all efforts to address this pandemic.
519 rows The FDA reissued the LabCorp COVID-19 RT-PCR Test emergency use. The Food and Drug Administration gave emergency approval to a COVID-19 antibody test that boasts. In some validation studies of these tests like the one FDA is conducting in partnership with NIH CDC and BARDA the samples used in addition to.
Sign up for our special edition newsletter to get a daily update on the coronavirus pandemic. SARS-CoV-2 antibody often referred to as serology tests look for antibodies in a sample to determine if an individual has had a past infection with the virus that causes COVID-19. FDA approves intravenous coronavirus antibody test Thomas Schinecker Roches head of diagnostics said the company aims to more than double production of tests Facebook.
While previous tests have measured antibody levels this one zeroes in on the antibodies that attack two specific aspects of the novel coronavirus.

Coronavirus Antibody Tests Fda Raises Standards Shots Health News Npr

Fda Increases Oversight Requirements For Covid 19 Antibody Tests Fiercebiotech
Antibody Testing Is Critical In Overcoming The Covid 19 Pandemic Now And In The Future
Antibody Test That Is Over 99 Accurate Gets Emergency Clearance By Fda
Posting Komentar untuk "What Coronavirus Antibody Tests Are Fda Approved"